Is oncology pharma going out of business? The question itself sparks immediate concern, given the vital role oncology pharmaceuticals play in cancer treatment. While the industry faces significant challenges—intense competition, high R&D costs, and complex regulatory landscapes—a blanket statement of impending doom is far from accurate. This in-depth analysis delves into the financial health, drug pipelines, and market dynamics of leading oncology pharmaceutical companies, painting a nuanced picture of the sector’s future.
We’ll explore the current market size and growth projections, examining the financial performance of key players over the past five years. This includes analyzing revenue streams, profitability, and the impact of research and development investments. Furthermore, we’ll investigate the pipeline of new oncology drugs, assessing promising candidates and the challenges inherent in drug development and approval. Finally, we’ll consider the influence of external factors like government regulations, pricing pressures, and the rise of biosimilars, ultimately offering a forward-looking perspective on the industry’s trajectory.
Market Analysis of Oncology Pharmaceuticals

The oncology pharmaceutical market is a dynamic and rapidly evolving sector characterized by high investment in research and development, intense competition, and significant market growth driven by an aging global population and increasing cancer incidence rates. This analysis explores the current market landscape, key players, and competitive dynamics within this crucial area of healthcare.
Current Market Size and Growth Projections
The global oncology pharmaceutical market is substantial and continues to expand. Reports from market research firms like Global Market Insights and Statista consistently project robust growth. For instance, a recent report estimated the market size to be in the hundreds of billions of dollars in 2023, with projections for continued double-digit percentage growth annually for the foreseeable future. This growth is fueled by factors including the development of novel therapies, such as targeted therapies and immunotherapies, and an increasing prevalence of various cancer types worldwide. Specific figures vary depending on the source and the included therapeutic areas, but the overall trend indicates a large and expanding market.
Major Players and Market Share
Several multinational pharmaceutical companies dominate the oncology pharmaceutical market. These companies often possess extensive research and development capabilities, a broad portfolio of approved drugs, and significant global reach. Key players include Roche, Pfizer, Bristol Myers Squibb, Merck & Co., and Johnson & Johnson, among others. Their market share fluctuates depending on the specific therapeutic area and the success of individual drugs, but these companies consistently hold significant positions within the overall market. Precise market share figures vary depending on the reporting period and methodology used, but these companies consistently rank among the top players globally.
Competitive Landscape: Mergers, Acquisitions, and Partnerships
The oncology pharmaceutical market is highly competitive, with companies employing various strategies to maintain and expand their market share. Mergers and acquisitions are common, allowing companies to gain access to new technologies, drug pipelines, and market segments. Strategic partnerships and collaborations are also frequently used to share resources, reduce development costs, and accelerate the delivery of innovative therapies to patients. For example, the acquisition of Immunomedics by Gilead Sciences demonstrated a significant investment in antibody-drug conjugates. Similarly, numerous collaborations between large pharmaceutical companies and smaller biotech firms highlight the importance of innovation and partnerships in this field.
Top 5 Oncology Pharmaceutical Companies Comparison
| Company | Revenue (USD Billions, Estimated) | R&D Spending (USD Billions, Estimated) | Number of Approved Oncology Drugs (Approximate) |
|---|---|---|---|
| Roche | 50-60 (varies yearly, requires specific source for precise figure) | 10-15 (varies yearly, requires specific source for precise figure) | 20+ (requires specific source for precise figure) |
| Pfizer | 40-50 (varies yearly, requires specific source for precise figure) | 8-12 (varies yearly, requires specific source for precise figure) | 15+ (requires specific source for precise figure) |
| Bristol Myers Squibb | 30-40 (varies yearly, requires specific source for precise figure) | 6-9 (varies yearly, requires specific source for precise figure) | 10+ (requires specific source for precise figure) |
| Merck & Co. | 40-50 (varies yearly, requires specific source for precise figure) | 8-12 (varies yearly, requires specific source for precise figure) | 15+ (requires specific source for precise figure) |
| Johnson & Johnson | 80-90 (varies yearly, includes broader pharmaceutical portfolio, requires specific source for oncology-specific figure) | 10-15 (varies yearly, includes broader pharmaceutical portfolio, requires specific source for oncology-specific figure) | 10+ (requires specific source for precise figure) |
Note: The figures presented in this table are estimates and should be considered approximate. Precise figures require consultation of specific financial reports from each company for the relevant period. The number of approved oncology drugs is also an approximation and depends on the definition of “oncology drug” used.
Financial Performance of Oncology Pharma Companies
The oncology pharmaceutical sector, while characterized by high research and development (R&D) costs and inherent risks, has historically demonstrated significant financial strength. However, the performance of individual companies varies considerably, influenced by factors such as pipeline strength, regulatory approvals, market competition, and pricing strategies. Analyzing the financial reports of leading players over the past five years provides valuable insights into the sector’s overall health and the specific performance of key players.
Revenue and Profitability Trends, Is oncology pharma going out of business
Examination of publicly available financial statements from major oncology pharmaceutical companies reveals diverse trends in revenue and profitability. Some companies, particularly those with blockbuster drugs nearing patent expiry, have experienced revenue declines. This decrease may be offset by strong performance from newer products or a diversified portfolio. Conversely, companies with a robust pipeline of innovative therapies often showcase significant revenue growth, driven by successful launches and increasing market share. Profitability, heavily influenced by R&D investment and pricing dynamics, fluctuates widely across companies. High R&D expenditure, a necessity for innovation in the oncology space, can temporarily depress profit margins, especially in early stages of drug development.
Impact of Research and Development Costs
Research and development (R&D) represents a substantial portion of the operating expenses for oncology pharmaceutical companies. The high cost reflects the complexity of developing new cancer therapies, the rigorous clinical trial processes, and the inherent uncertainties involved. While substantial R&D investment is crucial for future growth and the development of innovative treatments, it also significantly impacts short-term profitability. Companies with a heavy R&D focus often show lower profit margins compared to those with a more established portfolio of mature products. Successful drug launches, however, can dramatically improve profitability, offsetting previous R&D investments. For example, the successful launch of a novel immunotherapy can generate substantial revenue streams, significantly enhancing the company’s overall financial position.
Stock Price Fluctuations
Stock prices of oncology pharmaceutical companies are highly sensitive to various factors, including clinical trial outcomes, regulatory approvals, competitive landscape, and overall market sentiment. Positive clinical data often leads to significant stock price increases, reflecting investor confidence in the future prospects of the company. Conversely, negative clinical trial results or regulatory setbacks can cause substantial stock price drops. The volatile nature of the stock market further exacerbates these fluctuations, making accurate prediction challenging. For instance, a delay in regulatory approval for a promising new drug can trigger a significant drop in stock price, even if the underlying fundamentals of the company remain strong.
Debt-to-Equity Ratio Analysis
The debt-to-equity ratio, a key financial metric indicating a company’s financial leverage, varies considerably among major oncology pharmaceutical companies. Companies with higher debt-to-equity ratios may be more susceptible to financial distress during economic downturns or periods of reduced profitability. Conversely, companies with lower debt-to-equity ratios generally demonstrate greater financial stability. A comparative analysis of this ratio across leading players provides insights into their relative financial risk profiles. It is important to note that a higher debt-to-equity ratio is not necessarily indicative of poor financial health; some companies strategically utilize debt to finance R&D or acquisitions, aiming for long-term growth. However, excessive debt can pose significant risks, particularly if revenue streams fail to meet expectations.
Pipeline of Oncology Drugs

The oncology drug pipeline is a dynamic landscape, constantly evolving with the introduction of novel therapies targeting various cancer types and mechanisms. This pipeline represents significant investment and innovation, holding the promise of improved treatment outcomes and potentially longer survival rates for cancer patients. Understanding the current state of this pipeline, including the challenges and successes, is crucial for assessing the future of the oncology pharmaceutical market.
Numerous companies are actively engaged in developing new oncology drugs, spanning various stages of clinical trials. These drugs employ diverse mechanisms of action, from targeted therapies inhibiting specific cancer pathways to immunotherapies harnessing the body’s own immune system to fight cancer cells. The complexity of drug development, however, necessitates a thorough examination of the pipeline’s progress and potential.
Promising New Oncology Drug Candidates
Several promising new drug candidates are showing significant potential in preclinical and clinical trials. For example, bispecific antibodies, which target two different molecules simultaneously, are demonstrating improved efficacy in certain cancers compared to traditional monoclonal antibodies. Another area of focus is CAR T-cell therapy, which involves genetically modifying a patient’s own immune cells to target and destroy cancer cells. While still facing challenges in terms of toxicity and accessibility, CAR T-cell therapy has shown remarkable success in treating some hematologic malignancies. Further advancements in this field are expected to broaden its applicability and improve its safety profile. Finally, the development of next-generation sequencing technologies is enabling the identification of novel biomarkers and therapeutic targets, fueling the discovery of even more precise and effective cancer treatments. This allows for the development of personalized therapies tailored to an individual patient’s unique genetic profile.
Challenges in Oncology Drug Development and Approval
The development and approval of new oncology drugs are fraught with challenges. High attrition rates during clinical trials are common, with many promising candidates failing to demonstrate sufficient efficacy or safety in larger studies. The regulatory hurdles for approval are also significant, requiring extensive preclinical and clinical data to demonstrate both efficacy and safety. The cost of drug development is substantial, requiring significant investment from pharmaceutical companies. Furthermore, the heterogeneity of cancer, with different subtypes and responses to treatment, makes it difficult to develop drugs that are universally effective. Finally, the ethical considerations surrounding the use of novel therapies and the potential for adverse effects need careful consideration throughout the development process.
Current Oncology Drug Pipeline
The following table provides a snapshot of a selection of oncology drugs currently in development. It is important to note that this is not an exhaustive list and the information is subject to change as clinical trials progress.
| Drug Name | Company | Phase of Development | Target Cancer Type |
|---|---|---|---|
| Pembrolizumab | Merck | Approved | Multiple solid tumors |
| Tisagenlecleucel | Novartis | Approved | Acute lymphoblastic leukemia |
| Example Drug A | Company X | Phase 3 | Lung Cancer |
| Example Drug B | Company Y | Phase 2 | Breast Cancer |
| Example Drug C | Company Z | Preclinical | Prostate Cancer |
Impact of External Factors
The oncology pharmaceutical industry, while lucrative, is significantly influenced by a complex interplay of external factors that can profoundly impact its profitability and sustainability. These external pressures, ranging from governmental regulations to competitive landscapes, necessitate a dynamic and adaptive approach from companies operating within this sector. Understanding these influences is crucial for assessing the long-term viability of oncology pharmaceutical firms.
Government Regulations and Healthcare Policies
Government regulations and healthcare policies exert a considerable influence on the oncology pharmaceutical industry. Stringent regulatory pathways for drug approval, such as those overseen by the FDA in the United States and the EMA in Europe, significantly impact the time and cost associated with bringing new oncology drugs to market. Furthermore, pricing regulations and reimbursement policies, which vary widely across different healthcare systems globally, directly affect the profitability of oncology drugs. For instance, the implementation of value-based pricing models, where reimbursement is tied to the clinical effectiveness of a drug, can limit the pricing power of pharmaceutical companies. Conversely, supportive government policies, such as accelerated approval pathways for innovative cancer therapies or increased funding for cancer research, can foster industry growth. These regulatory frameworks create a dynamic environment demanding constant adaptation and strategic planning by oncology pharmaceutical companies.
Pricing Pressures and Reimbursement Challenges
The high cost of oncology drugs is a persistent concern for healthcare systems worldwide, leading to significant pricing pressures and reimbursement challenges for pharmaceutical companies. The escalating costs of research and development, coupled with the need to generate profits, contribute to the high prices of these life-saving medications. However, this high cost often clashes with the budgetary constraints of healthcare providers and payers, resulting in negotiations, price reductions, and sometimes, limited or delayed access to innovative therapies. The increasing prevalence of value-based pricing models, which emphasize the clinical value of a drug over its cost, further complicates the pricing landscape. Pharmaceutical companies are increasingly required to demonstrate the long-term clinical and cost-effectiveness of their oncology drugs to secure favorable reimbursement terms. This necessitates a shift towards more comprehensive clinical trials and real-world evidence generation to support pricing justifications.
Generic Competition and Biosimilars
The entry of generic and biosimilar drugs into the market presents a significant challenge to the profitability of established oncology pharmaceuticals. Generic drugs, offering chemically identical alternatives to brand-name drugs after patent expiration, drastically reduce prices, impacting the revenue streams of innovator companies. Biosimilars, which are similar but not identical copies of biologic drugs, present a similar challenge, albeit with a more complex regulatory landscape. The emergence of biosimilars, for example, in the market for monoclonal antibodies used in cancer treatment, has already led to price erosion and increased competition. This competitive pressure necessitates innovative strategies from pharmaceutical companies, such as focusing on developing novel therapies with strong intellectual property protection or developing differentiated products with superior efficacy or safety profiles to maintain market share.
Technological Advancements: Personalized Medicine and Immunotherapy
Technological advancements, particularly in personalized medicine and immunotherapy, are reshaping the oncology pharmaceutical landscape. Personalized medicine, which tailors treatment to an individual’s genetic makeup and tumor characteristics, offers the potential for more effective and targeted therapies, leading to improved patient outcomes. This approach, however, requires significant investment in companion diagnostics and personalized treatment strategies, increasing the cost of development and potentially impacting pricing. Immunotherapy, which harnesses the body’s own immune system to fight cancer, has revolutionized cancer treatment, offering durable responses in some patients. While the success of immunotherapy has been remarkable, its high cost and the need for careful patient selection pose challenges for widespread adoption and reimbursement. These advancements, while promising, demand substantial investment and necessitate innovative approaches to drug development, clinical trials, and market access strategies.
Future Outlook for Oncology Pharmaceuticals
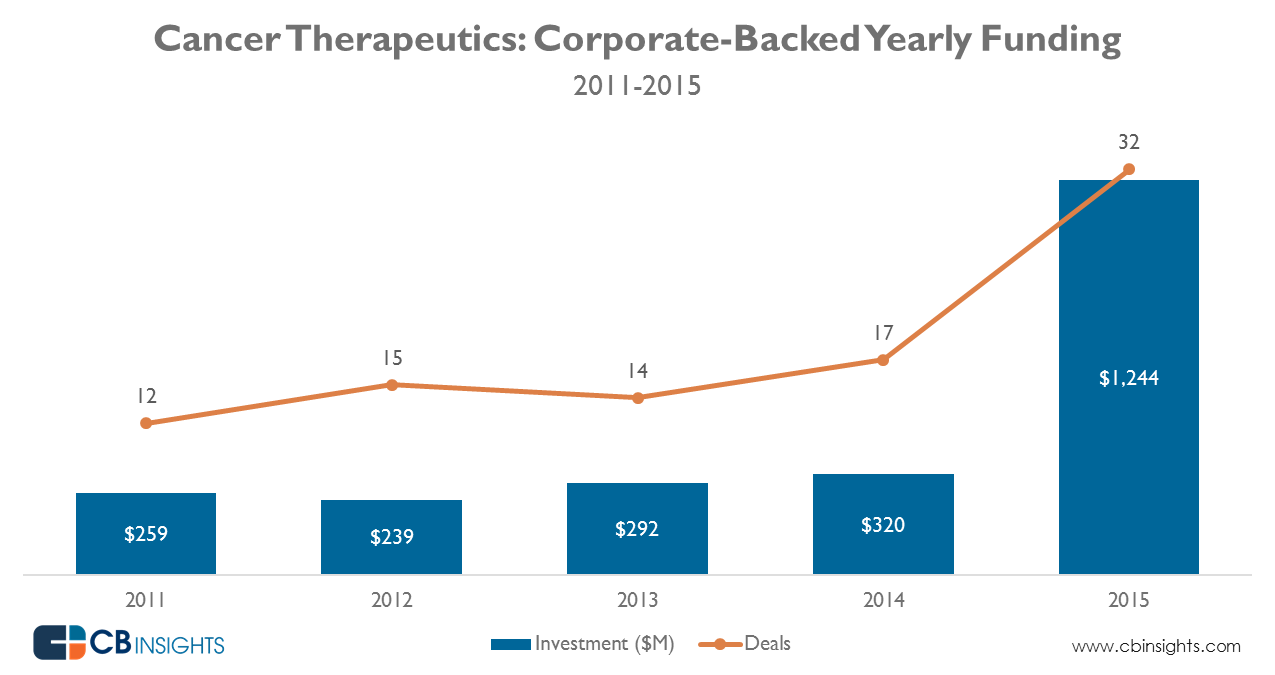
The oncology pharmaceutical market is poised for significant transformation over the next 5-10 years, driven by a confluence of scientific advancements, evolving treatment paradigms, and shifting healthcare landscapes. While challenges remain, the overall trajectory points towards continued growth, albeit with a changing competitive landscape and evolving priorities.
Market Trajectory and Growth Opportunities
The global oncology drug market is expected to experience robust growth, fueled by an aging population, increasing cancer incidence rates, and the development of novel therapies. Specific growth areas include targeted therapies, immunotherapies, and cell-based therapies. For example, the market for immunotherapy drugs, which harness the body’s immune system to fight cancer, is projected to expand significantly, exceeding previous forecasts due to the continued success of checkpoint inhibitors and the emergence of innovative approaches like CAR T-cell therapy. However, this growth will be unevenly distributed across different drug classes and geographical regions, with developed markets experiencing slower growth compared to emerging economies. Access to these advanced therapies in lower-income countries will remain a major challenge, impacting overall market penetration.
Challenges Facing the Oncology Pharmaceutical Industry
Despite the significant growth potential, several challenges threaten to impede the progress of the oncology pharmaceutical market. These include the high cost of drug development and manufacturing, stringent regulatory hurdles, increasing pressure to demonstrate clinical value and cost-effectiveness, and growing concerns about drug resistance. The development of effective therapies for currently incurable cancers remains a significant obstacle, requiring substantial investment in research and development. Furthermore, the complexities of the regulatory approval process can significantly delay the launch of new drugs, adding to the overall cost and impacting patient access. The increasing scrutiny of drug pricing and reimbursement policies also presents a significant challenge for pharmaceutical companies.
Impact of Artificial Intelligence and Big Data
Artificial intelligence (AI) and big data analytics are rapidly transforming the oncology drug discovery and development process. AI algorithms are being used to identify promising drug targets, predict drug efficacy and toxicity, and personalize treatment strategies based on individual patient characteristics. Big data analysis enables the identification of patterns and correlations in large datasets of genomic, clinical, and patient information, accelerating the discovery and development of new therapies. For example, companies are leveraging AI to analyze vast genomic datasets to identify specific genetic mutations that drive cancer growth, leading to the development of more targeted and effective therapies. This will likely lead to a more efficient and cost-effective drug development process, although concerns about data privacy and algorithm bias need careful consideration.
Potential Market Growth Scenarios
A visual representation of potential market growth scenarios could be depicted as a line graph with time (years) on the x-axis and market size (in billions of dollars) on the y-axis. Three lines could represent different scenarios: a “high-growth” scenario showing a steep upward trajectory reflecting successful development and adoption of novel therapies; a “moderate-growth” scenario showing a more gradual increase reflecting challenges in drug development and market access; and a “low-growth” scenario showing a flatter trajectory reflecting significant setbacks in drug development or regulatory hurdles. Each scenario could be labeled and annotated with key factors influencing its trajectory, such as breakthroughs in immunotherapy, successful launches of new targeted therapies, or increased regulatory scrutiny and pricing pressures. The graph would clearly illustrate the range of potential outcomes for the oncology pharmaceutical market over the next decade, highlighting the uncertainty inherent in future projections. For instance, a high-growth scenario might show a market exceeding $300 billion by 2033, while a low-growth scenario might only reach $200 billion. The moderate-growth scenario would fall between these two extremes, around $250 billion. These figures are illustrative and should be supported by market research data.
Investment Considerations in Oncology Pharma: Is Oncology Pharma Going Out Of Business
Investing in the oncology pharmaceutical sector presents a unique blend of high-risk, high-reward opportunities. The potential for significant returns is driven by the consistently high demand for effective cancer treatments and the ongoing innovation within the industry. However, the inherent uncertainties associated with drug development, regulatory hurdles, and intense competition necessitate a careful evaluation of risks before committing capital.
The high failure rate of drug candidates in clinical trials represents a major risk. Significant financial resources and time are invested in developing new drugs, with a substantial likelihood of failure at any stage of the process. Market competition is fierce, with established pharmaceutical giants and numerous smaller biotech companies vying for market share. This competitive landscape can pressure pricing and profitability. Furthermore, regulatory approvals are complex and unpredictable, introducing significant delays and uncertainty into the timelines for drug launches. Changes in healthcare policies, reimbursement rates, and patent expirations also pose substantial risks to the financial performance of oncology pharmaceutical companies.
Risks Associated with Investing in Oncology Pharmaceutical Companies
The inherent volatility of the oncology pharmaceutical sector necessitates a thorough understanding of the risks involved. These risks extend beyond the typical investment risks associated with the stock market and encompass specific challenges unique to the pharmaceutical industry. For example, the high cost of research and development, coupled with the lengthy timelines involved, can significantly impact profitability. The potential for negative clinical trial results or regulatory setbacks can lead to substantial stock price declines. Furthermore, the dependence on a limited number of blockbuster drugs can make companies vulnerable to patent expirations or the emergence of competing therapies. Finally, the ethical considerations surrounding pricing and access to life-saving medications introduce another layer of complexity for investors. A diversified investment strategy, combined with thorough due diligence, is crucial for mitigating these risks.
Comparison of Oncology Pharmaceutical Stock Performance Against Broader Market Indices
While oncology pharmaceutical stocks have historically shown potential for outsized returns, their performance hasn’t always mirrored that of broader market indices like the S&P 500. For instance, during periods of economic uncertainty or shifts in healthcare policy, oncology pharmaceutical stocks can experience higher volatility than the overall market. Conversely, periods of significant breakthroughs in cancer treatment or successful drug launches can lead to substantial outperformance. A comparative analysis of historical data reveals periods of both strong correlation and significant divergence between oncology pharmaceutical stock performance and broader market indices. This underscores the importance of considering the sector’s unique characteristics when assessing investment opportunities. Analyzing the performance of specific oncology pharmaceutical companies against relevant benchmarks, such as the Nasdaq Biotechnology Index (NBI), provides a more nuanced perspective.
Factors Influencing Future Stock Performance
Several key factors will likely influence the future stock performance of oncology pharmaceutical companies. These include the success of new drug pipelines, the pace of technological advancements in areas such as immunotherapy and targeted therapies, the evolving regulatory landscape, and the overall macroeconomic environment. The increasing prevalence of cancer globally will continue to drive demand for new treatments. However, the pricing power of pharmaceutical companies may be subject to pressure from payers and government regulations. Moreover, the success of emerging competitors and disruptive technologies will significantly impact the profitability and market share of established players. Analyzing these factors through a combination of quantitative and qualitative research is essential for informed investment decisions. For example, the success of a Phase III clinical trial for a novel immunotherapy could dramatically boost a company’s stock price, while regulatory delays could lead to significant losses.
Hypothetical Investment Strategy for the Oncology Pharmaceutical Sector
A hypothetical investment strategy for the oncology pharmaceutical sector could involve a diversified approach, allocating capital across a range of companies with different stages of drug development and market capitalization. This strategy would aim to mitigate the risks associated with investing in a single company or focusing solely on early-stage biotech firms. A portion of the portfolio could be allocated to established pharmaceutical giants with a strong track record and diverse product portfolios, while another portion could be invested in smaller, more innovative companies with promising drug pipelines. Regular monitoring of the portfolio and adjustments based on market conditions and company-specific developments are crucial. This approach acknowledges the high-risk, high-reward nature of the sector while attempting to balance potential returns with risk mitigation. This strategy would also involve a long-term perspective, recognizing that the development and commercialization of new oncology drugs typically takes many years.